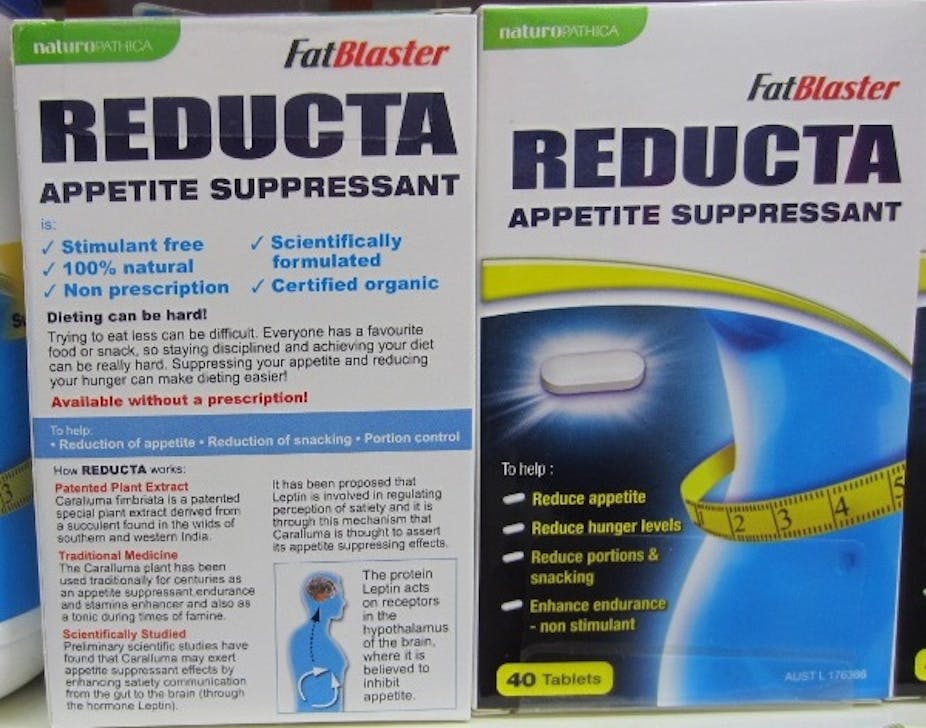
I submitted a complaint about the promotion of “FatBlaster Reducta” (ARTG no: 176366) to the Therapeutic Goods Administration in March 2011. The product is a “complementary medicine” containing an extract of Caralluma fimbriata (a succulent native to India), is sponsored by Pharmacare Laboratories, and promoted for weight loss.
And I submitted an additional complaint about the ongoing television promotion of this product in May 2011.
In August 2011, the Therapeutic Goods Advertising Complaints Resolution Panel (CRP) agreed the promotion breached many provisions of the Therapeutic Goods Advertising Code 2007 (determinations 2011-03-008 and 2011-05-008).
Pharmacare Laboratories was “requested” to withdraw the advertisements, the representations made and advise other parties promoting this product that their representations should also be withdrawn.
The CRP’s request included “to withdraw any representations that the advertised product or its ingredients have benefits in relation to weight management or weight control, reducing snacking, or aiding in eating less or reducing portion sizes” and “to withdraw the representations that the advertised product or its ingredients have benefits in relation to reducing feelings of hunger or suppressing appetite, except where those representations are clearly qualified as to the nature of the evidence supporting the claims, through clear statements that the claim is based only on evidence of traditional use.”
Pharmacare Laboratories declined to comply with the CRP’s request. So at the end of August 2011, the CRP recommended that the Therapeutic Goods Administration (TGA) should “order” the company to comply.
Still, the promotion of this product continued.
Over 12 months later, on October 9 2012, the TGA announced the cancellation of FatBlaster Reducta from the Australian Register of Therapeutic Goods (ARTG) on the grounds that there was insufficient evidence to support the indications for the product, the presentation of the product was unacceptable and the certifications made by the applicant regarding these matters were incorrect.
As of October 22 2012, this delisted product continues to be promoted. Pharmacy Online, for example, continues to make the same claims (and use the same video) that the CRP agreed breached the Therapeutic Goods Advertising Code.
The product also remains on the shelves of numerous pharmacies with no indication that it has been removed from the ARTG because “there was insufficient evidence to support the indications for the product.” These indications remain prominently displayed on the pack.
This is merely the latest example of the ongoing failure of the TGA and similar regulatory bodies to protect consumers from the claims made by those promoting and selling shonky “complementary medicines”.
There’s no pre-market assessment by the TGA of the claims made for listed complementary medicines. There’s only limited post-marketing surveillance and that has shown high levels of regulatory non-compliance.
It can take up to six months for a determination of the CRP to be made public and their “requests” to sponsors to withdraw misleading claims can be readily ignored – as in this case. It can take the TGA far longer to exhaust due process and cancel the product’s listing on the ARTG. And this doesn’t deter pharmacists and other retailers from clearing their stocks of delisted products through continued promotion and sales.
Promoting a delisted product is a breach of the Therapeutic Goods Act 1989 (s.42DL(1)(g)), the Therapeutic Goods Advertising Code 2007 (s.4(1)(a)) and for pharmacists, arguably the Health Practitioner Regulation National Law Act 2009 (s.133).
To date, complaints under the latter have proved unproductive.
On October 26 2011, for instance, I complained to the Australian Health Practitioner Regulation Agency (AHPRA) Pharmacy Board about the ongoing promotion of SensaSlim by pharmacists who continued making claims that had been shown to be false, misleading and deceptive by Federal Court Orders and CRP determinations.
I received an acknowledgement for the complaint from the Pharmacy Board on November 22, 2011. I subsequently pointed out to the Pharmacy Board that SensaSlim was delisted by the TGA on December 1 2011, but promotion by pharmacists continued.
I have yet to receive any further communication from the Board about these matters. I’ve now submitted further complaints to the Pharmacy Board about the ongoing promotion of FatBlaster Reducta by pharmacists.
Clearly, we need a more responsive regulatory system.
Sponsors such as Pharmacare Laboratories, which have had numerous upheld complaints about their products should be targeted for urgent post-market audit by the TGA, as recommended by the Australian National Audit Office Report. And it shouldn’t take 12 months or more to remove such products from the ARTG, especially if the audit has resulted from non-compliance with a CRP determination.
Notice of product delisting must be sent to the Pharmacy Guild, the Pharmaceutical Society of Australia and other retailers, advising them that continued promotion of such products is an offence.
Retailers should request the sponsor to remove delisted stock and provide reimbursement.
Finally, it would be helpful if the AHPRA Pharmacy Board wrote to all pharmacists reminding them of their obligations under under the Code of Conduct for Registered Health Practitioners (s.8.6), “All advertisements must conform to relevant consumer protection legislation”.
The National Law Act is also relevant. Subdivision 4, s.133 says, “a person must not advertise a regulated health service or a business that provides a regulated health service, in a way that: a) is false, misleading or deceptive or is likely to be misleading or deceptive; or b) creates an unreasonable expectation of beneficial treatment.”
We have complementary medicine companies (and pharmacists) who consistently ignore the rules, aided and abetted by bureaucratic red tape and toothless or sleeping tigers – the regulators. The result is that consumers get shafted. Surely, it’s time the government acted to protect its citizens.