Chronic kidney disease (CKD) is a significant and growing global public health problem. But the creation of a new type of stem cell offers new hope for therapies and drugs for this worldwide problem.
In Australia, approximately 13.4% of adults have chronic kidney disease (CKD), accounting for 1.7% of total direct health care expenditure. This has increased by a third over the last decade.
The prevalence of CKD continues to climb at a rate of 6% to 8% a year in Australia and is expected to increase further due to the ageing population and the growing epidemic of obesity-related diabetes.
The majority of people with CKD also have heart disease and kidney disease acts as a multiplier of morbidity, mortality and cost.
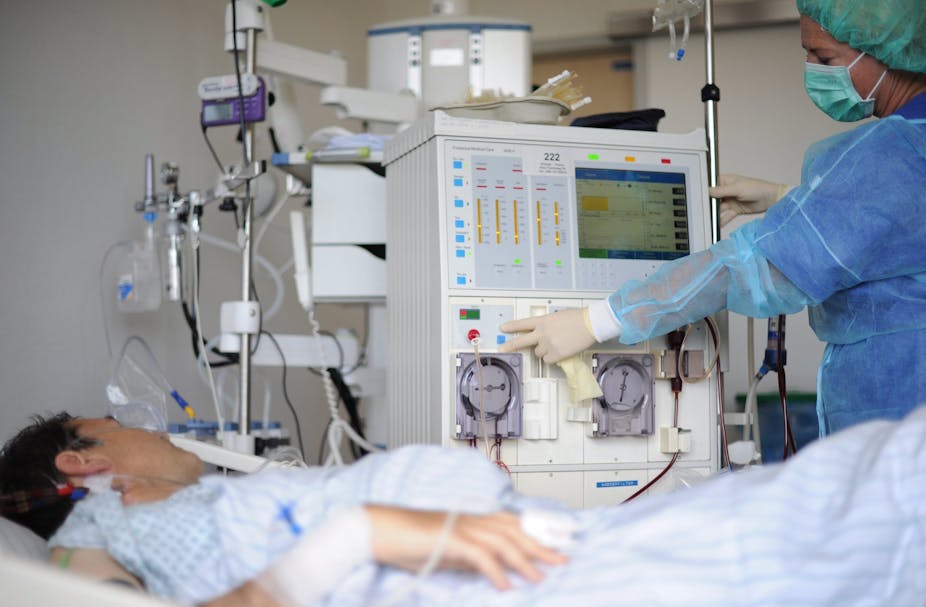
The principal treatments for patients with CKD, which leads to kidney failure, are dialysis and organ transplantation.
Given the poor quality of life from long-term dialysis treatment, the huge expenditure and the ever-increasing organ transplant waiting lists, there is an urgent need for more advanced therapeutic measures.
The promise of stem cell therapy
Stem cells are found in the early embryo, the foetus, amniotic fluid, placenta and umbilical cord blood.
The ability of stem cells to replace damaged or diseased cells and their potential to form kidney tissue is of great interest to those developing new therapeutic options for treating kidney disease.
It is currently not known if the kidney harbours a population of stem cells. However, significant “self-repair” and regeneration of the kidney are seen following acute or short-term injury.
After birth and for the rest of life, stem cells continue to reside in many sites of the body, including skin, hair follicles, bone marrow and blood, brain and spinal cord, the lining of the nose, gut, lung, joint fluid, muscle, and fat, to name a few.
In the growing body, stem cells are responsible for generating new tissues, and once growth is complete, they are responsible for repair and regeneration of damaged and ageing tissues.
A significant development in stem cell research occurred in 2007, when scientists announced they had developed a new technology that caused mature human cells to resemble pluripotent stem cells (iPS).
These iPS cells are similar in many ways to embryonic stem cells. But deriving iPS cells does not require embryos and is instead achieved by altering the gene activity within the mature cell.
In a international first, we have recently generated iPS cells generated from human kidney cells.
Specifically, we took kidney cells and reprogrammed them to become more immature embryonic-like cells that can be grown in the culture dish indefinitely.
Like embryonic stem cells, the kidney iPS cells can be changed into any cell type or tissue type in the body when grown in the culture dish under the right conditions.
This research is the stepping stone to the development of iPS cells from patients with genetic kidney disease, such as polycystic kidney disease (PKD) and Alport Syndrome.
Making iPS cells from kidneys represents a big advance in medicine, as there are currently limited options for studying human kidneys at the cellular level in culture.
Kidney iPS cells may maintain an epigenetic cell memory of the tissue from which they were derived. This would make them more able to produce kidney cells.
As the kidney iPS stem cells can divide indefinitely in a culture dish, we will attempt to make off-the-shelf mature kidney cells to use for drug testing and disease modelling.
This will enable us to understand kidney disease in a way we have never been able to before, advancing the potential of human iPS cells for modelling genetic kidney disorders and for screening new drug compounds.
In fact, iPS cells might one day provide a limitless source of replacement cells that can be grown from a patient’s own tissue.
These replacement would be genetically matched, therefore minimising the risk of rejection by the immune system.
Through the study of a patient’s iPS cell line, we could also discover information about that individual that would allow us to select or optimise that patient’s preventative and therapeutic care.
This will take personalised medicine to new heights and holds extraordinary potential for new drug discovery and safer medicines in the future.